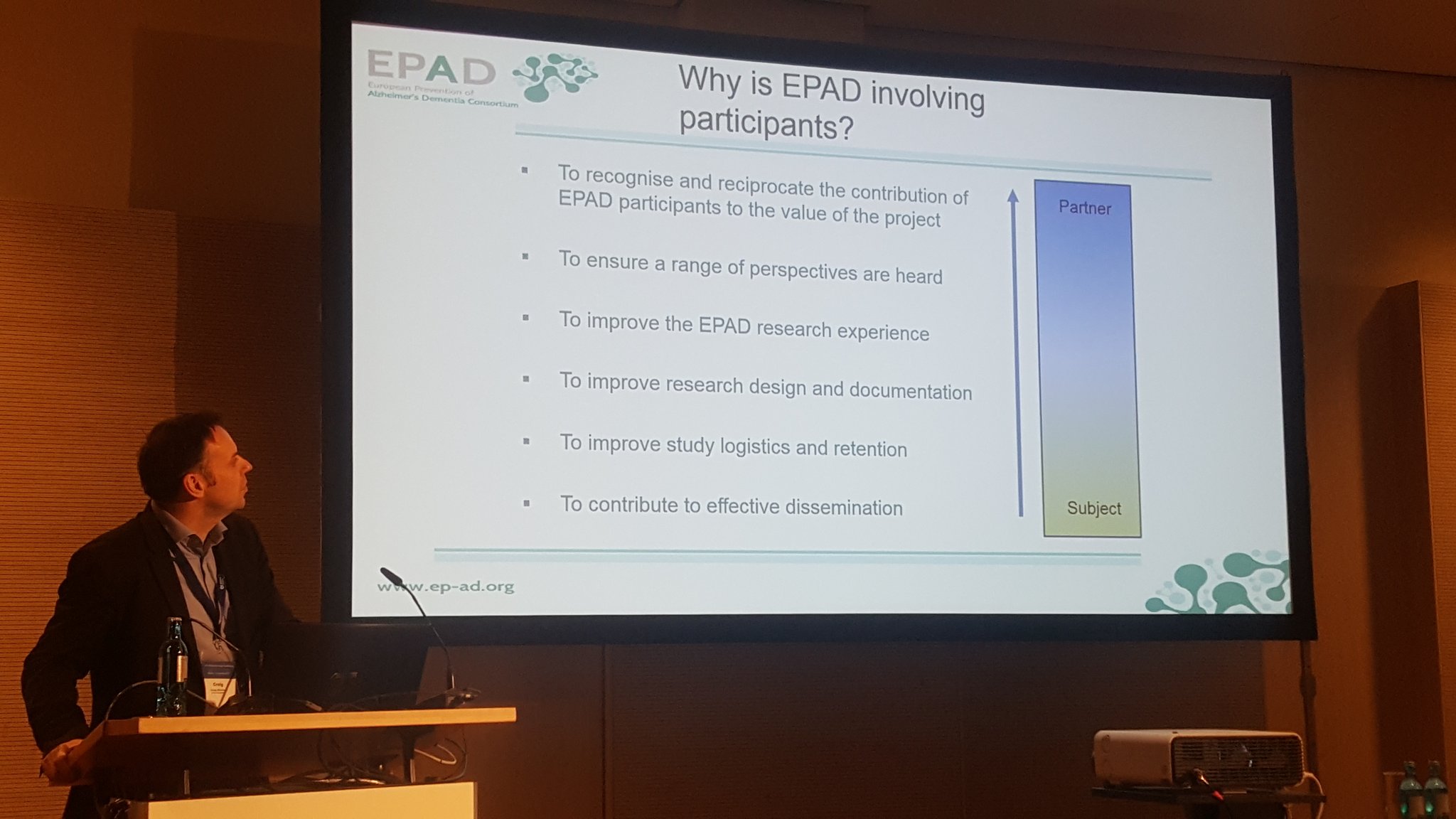
A great occasion to get to know more on three Alzheimer’s Disease IMI (Innovative Medicines Initiative) projects, was at the 27th Alzheimer Europe Conference (#27AEC), held in Berlin from 2-4 October. At a symposium convened by Eli Lilly, entitled: “Engaging with patient organisations within IMI consortia to inform quality, relevance and value in Alzheimer’s research – insights from MOPEAD, EPAD and ROADMAP”, the attendees were able to explore how these three initiatives are tackling AD across the disease continuum. The panelists provided their perspectives regarding the rationale for each project, engaging in a discussion on the concrete multipronged actions to improve timely diagnosis through citizens’ participation, pioneering novel approaches to clinical trials and providing evidence regarding the value of new medications. The first panelist was Mercé Boada, she is co-founder of Fundació ACE and Project Coordinator of the MOPEAD (Models of Patient Engagement for Alzheimer’s Disease). Her presentation was followed by an introduction to the EPAD (European Prevention of Alzheimer’s Disease) project by Craig Ritchie (pictured) Director of the Centre for Dementia Prevention. The presentations were rounded off by Catherine Reed principal research scientist at Eli Lilly who introduced ROADMAP (Real world Outcomes across the AD spectrum for better care: Multi-modal data Access Platform) and how real-world evidence may be used to address specific healthcare challenges. One of the highlights of the following discussions was the engagement of Pierre Meulien Executive Director of the IMI at the end of the symposium.
Watch the EPAD presentation on Engaging with patient organisations within IMI consortia to inform quality, relevance and value in Alzheimer’s research “ insights from MOPEAD, EPAD and ROADMAP” here.