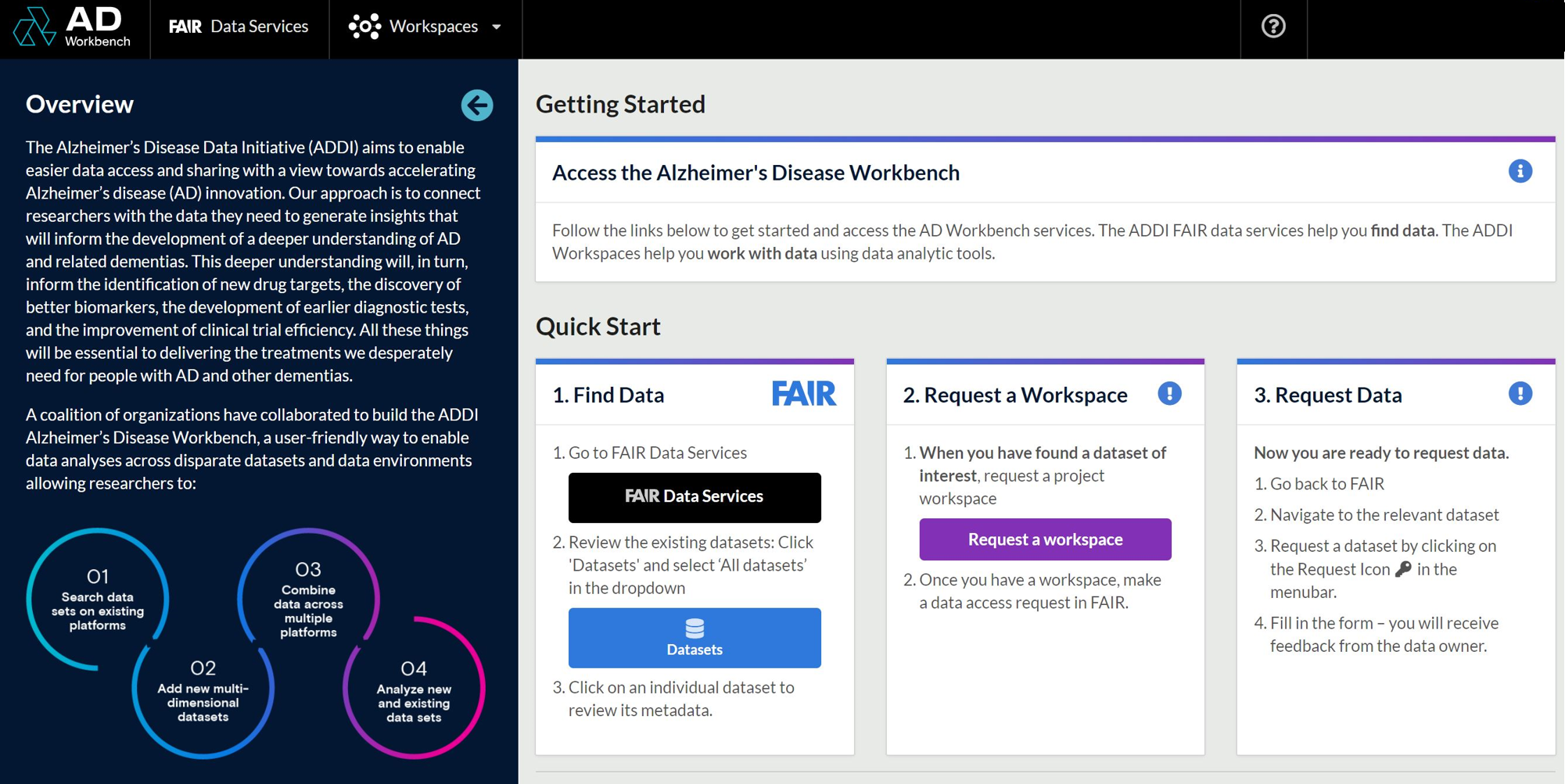
On 17 November 2020, a coalition of partners including the US Alzheimer’s Association, Gates Ventures and the Wellcome Trust launched the Alzheimer’s Disease Data Initiative (AD Data Initiative) and its Alzheimer’s Disease (AD) Workbench. Designed as a platform for federated data sharing, the AD Workbench aims to facilitate interoperability across existing data platform, by enabling researchers to import, pool and analyse datasets in a secure, cloud-based environment.
One year ago, the AD Data Initiative took its first steps on the path to being a trusted partner in the global data sharing movement. In their fall newsletter, the AD Data Initiative marked its one-year anniversary by sharing achievements, lessons learned along the way, and the work they still have left to do.
In just one year, the AD Data Initiative’s success can be reflected in numbers! Over 2,000 users from 80 countries, including users from 40 low- and middle-income countries, have engaged with the AD Workbench and have created more than 200 workspaces. These data contributing partners have made it possible for these researchers to access more than 35 datasets, with more than 50 new datasets in the development pipeline.
EPAD was the first project to place its entire dataset onto the Alzheimer’s Disease Workbench. From January 2015 until February 2020, the EPAD Longitudinal Cohort Study (LCS) screened over 2,000 participants across nine European countries to collect a wide range of cognitive, clinical, neuroimaging and biomarker data. A total of 2,096 participants were screened. From these 2,096 participants, EPAD followed up with 1,225 after one year, 421 after two years and 121 after three years. The latest and final EPAD dataset called Version.IMI. (V.IMI) is available in the AD Workbench to provide even greater value to the global neuroscience research community. To access the data, you will need to make an online request via the AD Workbench here.